A piston–cylinder device initially contains 0.A m3 (A is the digit in your SID ****ABC, if A is 0 or 1, then take the next higher digit between B or C) of air at 100 kPa and 80 °C.
Element 011: MOD002684 (Thermofluids) Assessment
QUESTIONS: Element 011: MOD002684 (Thermofluids) Assessment
1.
Part A
A piston–cylinder device initially contains 0.A m3 (A is the digit in your SID ****ABC, if A is 0 or 1, then take the next higher digit between B or C) of air at 100 kPa and 80 °C. The air is then compressed to 0.1 m3 in such a way that PV = constant. Determine the work done during this process. (5 Marks)
Part B
The air enters the compressor at a temperature of 280 K with an enthalpy of 280.13 kJ/kg and is compressed steadily to 400 K with an enthalpy of 400.98 kJ/kg. The mass flow rate of the air is 0.0A kg/s (A is the digit in your SID ****ABC, if A is 0, then take the next higher digit between B or C) and a heat loss of 16 kJ/kg occurs during the process. Determine the necessary power input to the compressor when:
a) the changes in kinetic and potential energies are negligible. (7 Marks)
b) the air velocity at entry is 100 m/s and exit is 150 m/s but potential energy is negligible. (7 Marks)
Provide a reflective commentary underpinning the KEY conceptual elements in solving problem Q1 Part B (maximum 100 words) (6 Marks
2.
Part A:
Heat is transferred to a heat engine from a furnace at a rate of 100 MW. If the rate of waste heat rejection to a nearby river is BC MW (BC are the digits in your SID ****ABC). Determine the net power output and the thermal efficiency for this heat engine. (8 Marks)
Part B
A heat source at 800 K loses 2000 kJ of heat to a sink at (a) 500 K and (b) ABC K (ABC are the digits in your SID ****ABC, for those who has SID number A = 0 or 1 or 2, replace A by 7) (shown in Figure Q2B). Determine which heat transfer process is more irreversible based on the entropy calculation. (11 Marks)
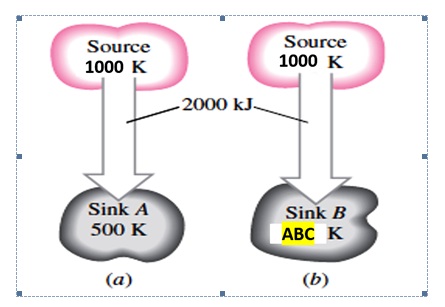
Figure Q2B
Provide a reflective commentary underpinning the KEY conceptual elements in solving problem Q1 Part B (maximum 100 words)
(6 Marks)
3.
An ideal Otto cycle has a compression ratio of 8. At the beginning of the compression process, air is at 100 kPa and 17°C, and ABC kJ/kg (ABC are the digits in your SID ****ABC, for those who has SID number A = 0 or 1 or 2, replace A by 7) of heat is transferred to air during the combustion process. Determine:
a) the pressure and temperature at the end of the compression process (8 Marks)
b) the thermal efficiency of the cycle (3 Marks)
c) the net output work of the cycle per kg of air (4 Marks)
d) the mean effective pressure for the cycle (4 Marks)
Assume Cv=0.718 kJ/kg.K and Cp=1.005 kJ/kg.K for air.
Provide a reflective commentary underpinning the KEY conceptual elements in solving this problem (maximum 100 words)(6 Marks)
4.
A fuel with a chemical formula C8H1A (A is the digit in your SID ****ABC, if A is an odd number, choose the next even number) is burned with dry air. The volumetric analysis of the exhaust products gases on a dry basis are CO2: 10.02%, O2: 5.62%, CO: 0.88% and N2: 83.48%.
Determine
- the air–fuel ratio
(15 Marks)
- the percentage of theoretical air used (4 Marks)
Provide a reflective commentary underpinning the KEY conceptual elements in solving this problem (maximum 100 words)(6 Marks)
5. Element 011: MOD002684 (Thermofluids) Assessment
Part A: Choose the correct option.
5A1. For the same compression ratio, the efficiency of diesel cycle is........Otto cycle (provide reason)
- Greater than
ii. Less than
iii. Equal to
iv. None of the above
(2 Marks)
5A2: Which of the following is true for a steady flow system? (provide reason)
i. mass entering = mass leaving
ii. mass does not enter or leave the system
iii. mass entering can be more or less than the mass leaving
iv. none of the mentioned
(2 Marks)
Part B
A rigid tank contains BC kg (BC is the digit in your SID ****ABC) of water at 90°C. If 80% by mass of the water is in the liquid form and the rest is in the vapour form. Determine
- the pressure in the tank (2 Marks)
- the dryness fraction of the mixture (3 Marks)
- the total volume of the tank (3 Marks)
- the internal energy (U) (3 Marks)
- the enthalpy (H) (2 Marks)
- the entropy (S) (2 Marks)
Provide a reflective commentary underpinning the KEY conceptual elements in solving problem Q5 Part B (maximum 100 words)
(6 Marks)
6. Element 011: MOD002684 (Thermofluids) Assessment
A certain gas of 2 kg is kept at BC (BC is the digit in your SID ****ABC) °C in a constant volume chamber of 0.3 m3 capacity. Heat is transferred to the gas until the temperature is of the gas reached to100 °C. The gas has cp = 1.968 kJ/kg-K and cv = 1.507 kJ/kg-K. Universal gas constant 8.314 kJ/kg-mol K. Determine:
- the molecular weight (3 Marks)
- the gas constant (2 Marks)
- the work done (3 Marks)
- the heat transferred (2 Marks)
- the change in internal energy (3 Marks)
- the change in enthalpy (3 Marks)
- the change in entropy
(3 Marks)
Provide a reflective commentary underpinning the KEY conceptual elements in solving this problem (maximum 100 words)
(6 Marks)
Element 011: MOD002684 (Thermofluids) Assessment
100% Plagiarism Free & Custom Written,
tailored to your instructions